Healthcare providers* considering testing people with possible COVID-19 should work with their local and state health departments to coordinate testing through public health laboratories, or work with commercial or clinical laboratories using viral tests granted an Emergency Use Authorization (EUA) by the U.S. Food and Drug Administration. CDC has guidance for who should be tested, but decisions about who should be tested are at the discretion of state and local health departments and/or healthcare providers. Testing for other pathogens by the provider should be done as part of the initial evaluation, as indicated, but should not delay testing for SARS-CoV-2, the virus that causes COVID-19.
Positive SARS-CoV-2 results should be reported. Read CDC’s Data and Reporting FAQ for Laboratories.
Clinical laboratories should NOT attempt viral isolation from specimens collected from people suspected to have COVID-19 unless this is performed in a BSL-3 laboratory.
*Pharmacists are considered healthcare providers in this guidance.
Specimen Type and Priority
All testing for SARS-CoV-2 should be conducted in consultation with a healthcare provider. Specimens should be collected as soon as possible once a decision has been made to pursue testing, regardless of the time of symptom onset. The guidance below addresses options for collection of specimens.
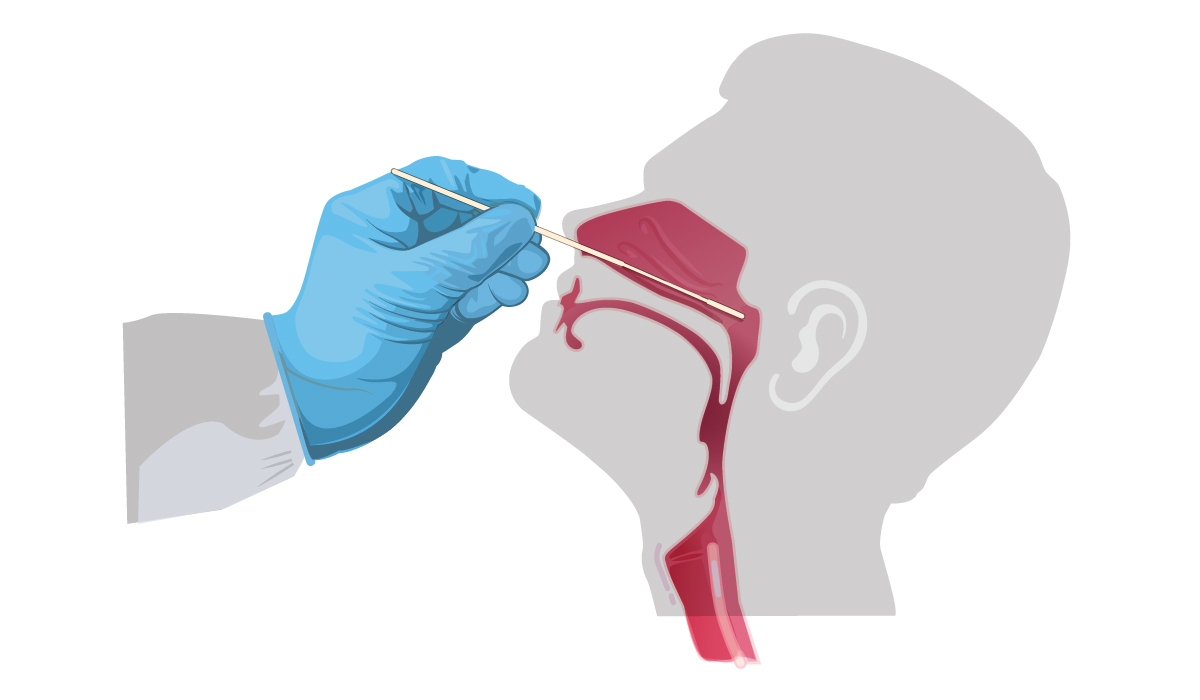
For initial diagnostic testing for SARS-CoV-2, CDC recommends collecting and testing an upper respiratory specimen. The following are acceptable specimens:
- A nasopharyngeal (NP) specimen collected by a healthcare provider; or
- An oropharyngeal (OP) specimen collected by a healthcare provider; or
- A nasal mid-turbinate swab collected by a healthcare provider or by a supervised onsite self-collection (using a flocked tapered swab); or
- An anterior nares (nasal swab) specimen collected by a healthcare provider or by home or supervised onsite self-collection (using a flocked or spun polyester swab); or
- Nasopharyngeal wash/aspirate or nasal wash/aspirate (NW) specimen collected by a healthcare provider.
Swabs should be placed immediately into a sterile transport tube containing 2-3mL of either viral transport medium (VTM), Amies transport medium, or sterile saline, unless using a test designed to analyze a specimen directly, (i.e., without placement in VTM), such as some point-of-care testsexternal icon. If VTM is not available, see the standard operating procedure for public health labs to create viral transport mediumpdf icon in accordance with CDC’s protocol.
The NW specimen and the non-bacteriostatic saline used to collect the specimen should be placed immediately into a sterile transport tube.
Testing lower respiratory tract specimens is also an option. For patients who develop a productive cough, sputum should be collected and tested for SARS-CoV-2. The induction of sputum is not recommended. When under certain clinical circumstances (e.g., those receiving invasive mechanical ventilation), a lower respiratory tract aspirate or bronchoalveolar lavage sample should be collected and tested as a lower respiratory tract specimen.
Collecting and Handling Specimens Safely
For providers collecting specimens or within 6 feet of patients suspected to be infected with SARS-CoV-2, maintain proper infection control and use recommended personal protective equipment (PPE), which includes an N95 or higher-level respirator (or facemask if a respirator is not available), eye protection, gloves, and a gown, when collecting specimens.
For providers who are handling specimens, but are not directly involved in collection (e.g. self-collection) and not working within 6 feet of the patient, follow Standard Precautions. Healthcare personnel are recommended to wear a form of source control (facemask or cloth face covering) at all times while in the healthcare facility.
PPE use can be minimized through patient self-collection while the healthcare provider maintains at least 6 feet of separation.
Handling Bulk-Packaged Sterile Swabs Properly for Upper Respiratory Sample Collection
Sterile swabs for upper respiratory specimen collection may be packaged in one of two ways:
- Individually wrapped (preferred when possible)
- Bulk packaged
Bulk-packaged swabs may be used for sample collection; however, care must be exercised to avoid SARS-CoV-2 contamination of any of the swabs in the bulk-packaged container.
- Before engaging with patients and while wearing a clean set of protective gloves, distribute individual swabs from the bulk container into individual disposable plastic bags.
- If bulk-packaged swabs cannot be individually packaged:
- Use only fresh, clean gloves to retrieve a single new swab from the bulk container.
- Close the bulk swab container after each swab removal and leave it closed when not in use to avoid inadvertent contamination.
- Store opened packages in a closed, airtight container to minimize contamination.
- Keep all used swabs away from the bulk swab container to avoid contamination.
- As with all swabs, only grasp the swab by the distal end of the handle, using gloved hands only.
- When patients are self-collecting their swabs under clinical supervision:
- Hand a swab to the patient only while wearing a clean set of protective gloves.
- The patient can then self-swab and place the swab in transport media or sterile transport device and seal.
- If the patient needs assistance, you can help the patient place the swab into transport media or a transport device and seal it.
General Guidelines
Proper collection of specimens is the most important step in the laboratory diagnosis of infectious diseases. A specimen that is not collected correctly may lead to false negative test results. The following specimen collection guidelines follow standard recommended procedures. For more information, including illustrations and step-by-step guidance, see the CDC Influenza Specimen Collectionpdf icon instructions. Note that these instructions are applicable for respiratory viruses in general, and not specific for only influenza virus.
I. Respiratory Specimens
A. Upper respiratory tract
Nasopharyngeal swab/Oropharyngeal (Throat) swab
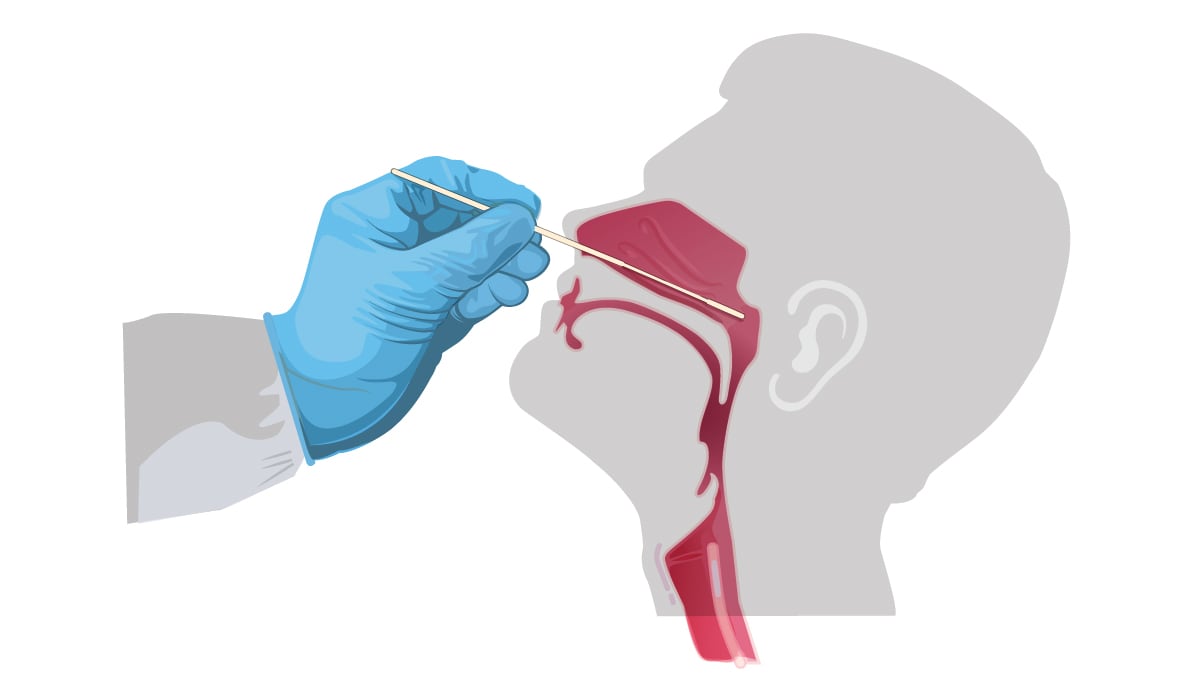
Use only synthetic fiber swabs with plastic or wire shafts. Do not use calcium alginate swabs or swabs with wooden shafts, as they may contain substances that inactivate some viruses and inhibit PCR testing. CDC is now recommending collecting only the NP swab, although OP swabs remain an acceptable specimen type. If both NP and OP swabs are collected, they should be combined in a single tube to maximize test sensitivity and limit use of testing resources.
NP swab: Insert minitip swab with a flexible shaft (wire or plastic) through the nostril parallel to the palate (not upwards) until resistance is encountered or the distance is equivalent to that from the ear to the nostril of the patient, indicating contact with the nasopharynx. Swab should reach depth equal to distance from nostrils to outer opening of the ear. Gently rub and roll the swab. Leave swab in place for several seconds to absorb secretions. Slowly remove swab while rotating it. Specimens can be collected from both sides using the same swab, but it is not necessary to collect specimens from both sides if the minitip is saturated with fluid from the first collection. If a deviated septum or blockage create difficulty in obtaining the specimen from one nostril, use the same swab to obtain the specimen from the other nostril.
OP swab: Insert swab into the posterior pharynx and tonsillar areas. Rub swab over both tonsillar pillars and posterior oropharynx and avoid touching the tongue, teeth, and gums.
Nasal mid-turbinate (NMT) swab, also called Deep Nasal Swab
Use a flocked tapered swab. Tilt patient’s head back 70 degrees. While gently rotating the swab, insert swab less than one inch (about 2 cm) into nostril (until resistance is met at turbinates). Rotate the swab several times against nasal wall and repeat in other nostril using the same swab.
Anterior nares specimen
Using a flocked or spun polyester swab, insert the swab at least 1 cm (0.5 inch) inside the nostril (naris) and firmly sample the nasal membrane by rotating the swab and leaving in place for 10 to 15 seconds. Sample both nostrils with same swab.
Nasopharyngeal wash/aspirate or nasal wash/aspirate
Attach catheter to suction apparatus. Have the patient sit with head tilted slightly backward. Instill 1 mL-1.5 mL of non-bacteriostatic saline (pH 7.0) into one nostril. Insert the tubing into the nostril parallel to the palate (not upwards). Catheter should reach depth equal to distance from nostrils to outer opening of ear. Begin gentle suction/aspiration and remove catheter while rotating it gently. Place specimen in a sterile viral transport media tube.
B. Lower respiratory tract
Bronchoalveolar lavage, tracheal aspirate, pleural fluid, lung biopsy
Collect 2-3 mL into a sterile, leak-proof, screw-cap sputum collection cup or sterile dry container.
Due to the increased technical skill and equipment needs, collection of specimens other than sputum from the lower respiratory tract may be limited to patients presenting with more severe disease, including people admitted to the hospital and/or fatal cases.
Sputum
Educate the patient about the difference between sputum and oral secretions (saliva). Have the patient rinse the mouth with water and then expectorate deep cough sputum directly into a sterile, leak-proof, screw-cap collection cup or sterile dry container.
II. Storage
Store specimens at 2-8°C for up to 72 hours after collection. If a delay in testing or shipping is expected, store specimens at -70°C or below.
III. Shipping
Samples may be shipped to CDC if repeated testing results remain inconclusive or if other unusual results are obtained. Please contact CDC at respvirus@cdc.gov prior to submitting samples.
If shipping samples to CDC: If specimens will ship without delay, store specimens at 2-8°C, and ship overnight to CDC on ice pack. If a delay in shipping will result in receipt at CDC more than 72 hours after collection, store specimens at -70°C or below and ship overnight to CDC on dry ice. Additional useful and detailed information on packing, shipping, and transporting specimens can be found at Interim Laboratory Biosafety Guidelines for Handling and Processing Specimens Associated with Coronavirus Disease 2019 (COVID-19).
Specimens must be packaged, shipped, and transported according to the current edition of the International Air Transport Association (IATA) Dangerous Goods Regulations external iconexternal icon.
Label each specimen container with the patient’s ID number (e.g., medical record number), unique CDC or state-generated nCov specimen ID (e.g., laboratory requisition number), specimen type (e.g., serum) and the date the sample was collected. Complete a CDC Form 50.34 for each specimen submitted. In the upper left box of the form, 1) for test requested select “Respiratory virus molecular detection (non-influenza) CDC-10401” and 2) for At CDC, bring to the attention of enter “Unit 84 (Non-flu Resp Virus)”.
Please refer to our instruction guidance for submitting CDC Form 50.34 found here: Guidelines For Submitting Specimens to CDCpdf icon.
For additional information, consultation, or the CDC shipping address, contact the CDC Emergency Operations Center (EOC) at 770-488-7100.
Article source: https://www.cdc.gov/coronavirus/2019-ncov/lab/guidelines-clinical-specimens.html